GxP - GMP, GDP, GLP, GCP: Definitions and Key Elements
GxP is a general acronym that encompasses regulations and guidelines governing good practices across various sectors. The "x" in GxP represents a specific area of application. In the pharmaceutical, biotechnology, and medical device industries, particular focus is on
- GMP (Good Manufacturing Practices),
- GDP (Good Distribution Practices),
- GLP (Good Laboratory Practices),
- GCP (Good Clinical Practices).
Each of these domains is essential to ensuring the quality, safety, and efficacy of pharmaceutical products throughout their lifecycle, from research to distribution.

Good Manufacturing Practices (GMP)

GMP are based on guidelines from the U.S. Food and Drug Administration (FDA) and regulate the manufacturing of pharmaceuticals and other medical products to ensure they are consistently produced according to stringent quality standards. These practices aim to prevent contamination, manufacturing errors, or other issues that could affect the final product's quality.
GMP are enforced by regulatory agencies globally, such as the FDA (Food and Drug Administration), EMA (European Medicines Agency), and WHO (World Health Organization).
Key elements of GMP:
- Quality Control: Every stage of pharmaceutical manufacturing must be monitored with regular testing and inspections of raw materials, intermediate products, and finished goods.
- Process Validation: Production, cleaning, and maintenance processes must be validated to ensure that the product meets the required specifications.
- Personnel Training: All personnel involved in production must be adequately trained in procedures and quality standards.
- Traceability: GMP requires comprehensive and precise documentation to trace each production batch, enabling a full history of manufacturing and distribution to be followed in case of any issues.
Good Distribution Practices (GDP)
GDP focuses on the distribution of pharmaceutical products. The aim of GDP is to prevent product deterioration, minimize the risk of falsification, and ensure that medications are transported and stored under appropriate conditions.
Key elements of GDP:
- Storage and Transport: Products must be stored under strictly controlled temperature and humidity conditions. Monitoring systems and mapping of storage areas, for instance, are recommended to ensure compliance with set standards.
- Traceability: Every batch of medicine or pharmaceutical product must be traceable from manufacturing to patient delivery, allowing easy recall and ensuring no counterfeit products enter the legitimate supply chain.
- Personnel Training and Qualification: Distributors must ensure that personnel at every stage of distribution are properly trained to handle products and adhere to GDP procedures.
- Complaints and Recalls: Procedures must be in place to address complaints and organize timely and accurate recalls if needed.

Good Laboratory Practices (GLP)

GLP governs non-clinical safety studies for pharmaceuticals and other regulated products. These guidelines ensure that laboratory data are reliable, verifiable, and reproducible, making it possible to confidently use study results in market authorization applications.
Key elements of GLP:
- Study Organization: GLP requires a clear definition of roles and responsibilities for every member of the research team, along with a detailed study plan.
- Documentation: All activities and observations must be recorded in real-time and rigorously documented to allow complete traceability of experimental data.
- Facilities and Equipment: Laboratories must be designed and maintained to minimize contamination risks. All equipment must be regularly maintained and calibrated according to compliant procedures to ensure reliable results.
- Data Archiving: Data must be securely stored for audits by the relevant regulatory authorities.
Good Clinical Practices (GCP)
GCP applies to clinical trials conducted on human subjects, ensuring the rights, safety, and well-being of participants while guaranteeing that the data collected are reliable and accurate. GCP is crucial for phases I-IV clinical trials that evaluate the efficacy and side effects of a drug before market release.
Key elements of GCP:
- Informed Consent: Participants must be informed about all aspects of the clinical trial and must provide their consent freely before participation.
- Study Protocol: Each clinical trial must follow a strict protocol approved by ethics committees, detailing the study objectives, eligibility criteria, and evaluation methods.
- Data Quality: Collected data must be complete and accurate. Audits may be conducted to verify the integrity of the data collected during trials.
- Monitoring and Risk Management: Clinical trials must be continuously monitored to quickly identify risks or incidents that could affect participant safety or the validity of the results.
GCP is coordinated by intergovernmental organizations such as the International Council for Harmonisation (ICH), which publishes guidelines recognized by most regulatory agencies worldwide, including the FDA and EMA.

GxP Pharma: The Importance of Environmental Monitoring
Environmental monitoring is a critical aspect of GxP to ensure the quality of pharmaceutical products throughout their lifecycle. Dickson is a globally recognized expert in manufacturing, implementing, and maintaining environmental monitoring solutions. Our data loggers, software, mapping solutions, calibration laboratories, and rigorous procedures play a key role in helping pharmaceutical companies maintain GxP compliance, particularly regarding temperature, humidity, and other critical parameters like carbon dioxide (CO2) monitoring.
Dickson’s Expertise in GxP Environmental Monitoring
Dickson offers a comprehensive range of products and services designed to meet the stringent requirements of GMP, GDP, GLP, and GCP guidelines. These solutions include connected data loggers, real-time monitoring and alert systems, accredited calibration laboratories, and data management software, all designed to deliver optimal accuracy and reliability in critical environments. By using our solutions, you can continuously monitor and document environmental parameters in your production, storage, and distribution sites.
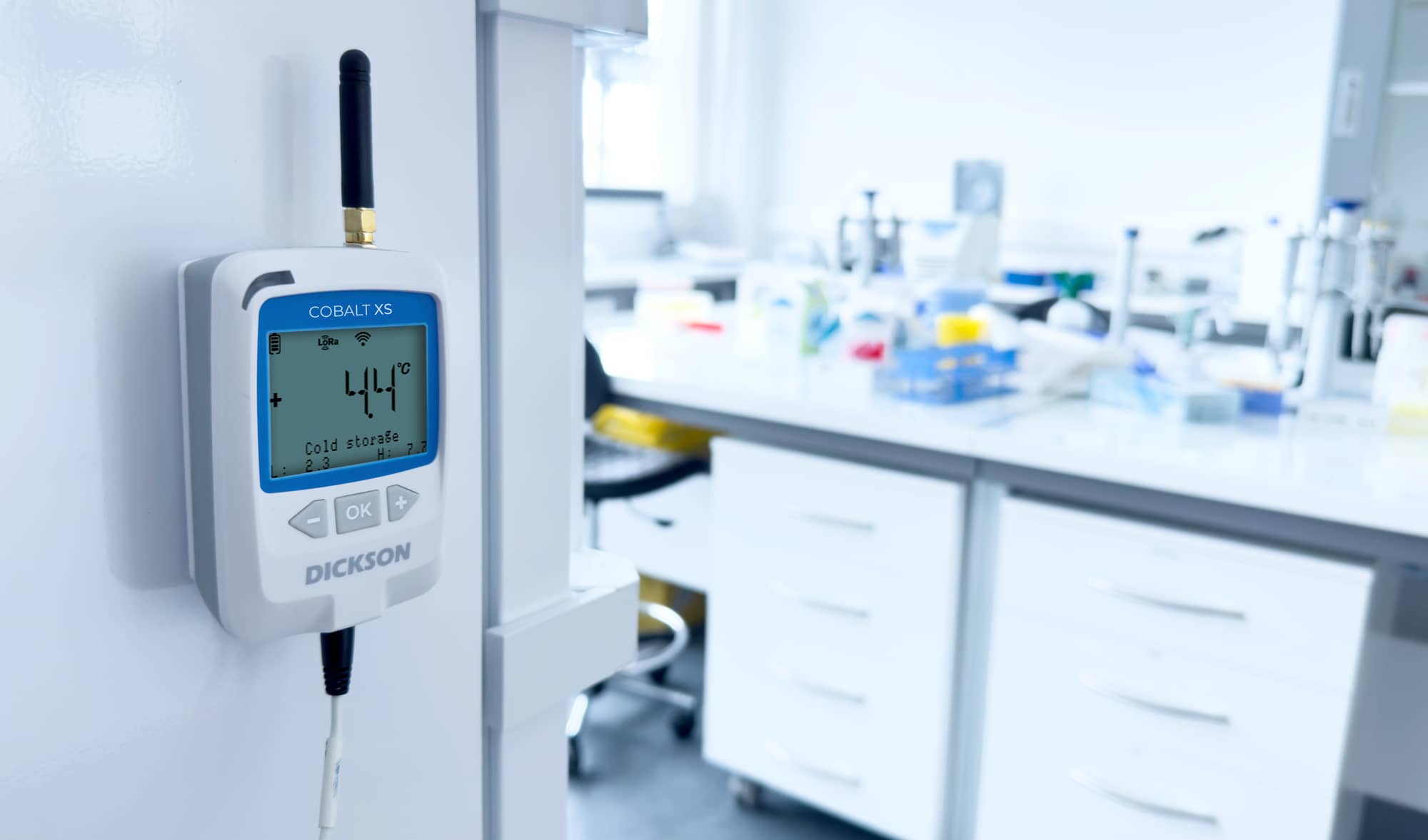
Temperature monitoring
Dickson solutions enable real-time temperature mapping and monitoring in sensitive areas such as cold rooms, pharmaceutical warehouses, incubators, clean rooms, and laboratory refrigerators. These monitoring systems are essential to ensure that temperature-sensitive products (vaccines, biological samples, injectable drugs, etc.) remain within conditions compliant with stability studies. With cutting-edge technology, Dickson’s data loggers can collect, record, and transmit accurate data over extended periods, reducing the risks associated with temperature excursions.
Humidity Monitoring
Dickson recorders can include probes and humidity sensors, another critical parameter for sensitive products. Our monitoring systems and mapping solutions ensure that humidity conditions meet the storage and production requirements set by GMP, minimizing the risk of product degradation.
CO2 and Other Parameters
For production or laboratory environments that require strict CO2 control, such as cell culture incubators, Dickson monitoring solutions integrate high-precision sensors that ensure compliance with GLP and GMP standards. The flexibility of Dickson’s monitoring systems allows for customization according to the specific requirements of each lab or production site.

Managing Excursions with Dickson Monitoring Systems
One of the major challenges for pharmaceutical companies is managing temperature, humidity, or CO2 excursions. Dickson’s real-time monitoring systems are designed to quickly detect any excursions and trigger automatic alerts, allowing responsible personnel to intervene before the product is compromised.
Real-time Alerts:
Dickson systems are equipped with alert mechanisms that immediately notify teams when environmental conditions exceed predefined ranges. Alerts can be configured to trigger visual and audible alarms or send notifications via SMS or email, ensuring quick and effective responses to prevent product loss.
Compliance Documentation and Traceability with Dickson Expertise
Accurate and compliant documentation of environmental monitoring and corrective actions is essential under GxP. Dickson has developed advanced software solutions—DicksonOne and OCEAView—that centralize the collection and processing of environmental data, simplifying traceability and audit management.

Contact Dickson’s GxP Compliance Experts
Dickson’s expertise in environmental monitoring and excursion management significantly contributes to the GxP compliance of many companies in the pharmaceutical sector.




